Higher chemistry assignment mole
Chemistry Teaching Resources - CfE New Higher Chemistry
Documents Flashcards Grammar checker. In its absence the protein, collagen, cannot form fibres properly and this results in skin lesions and blood vessel fragility.
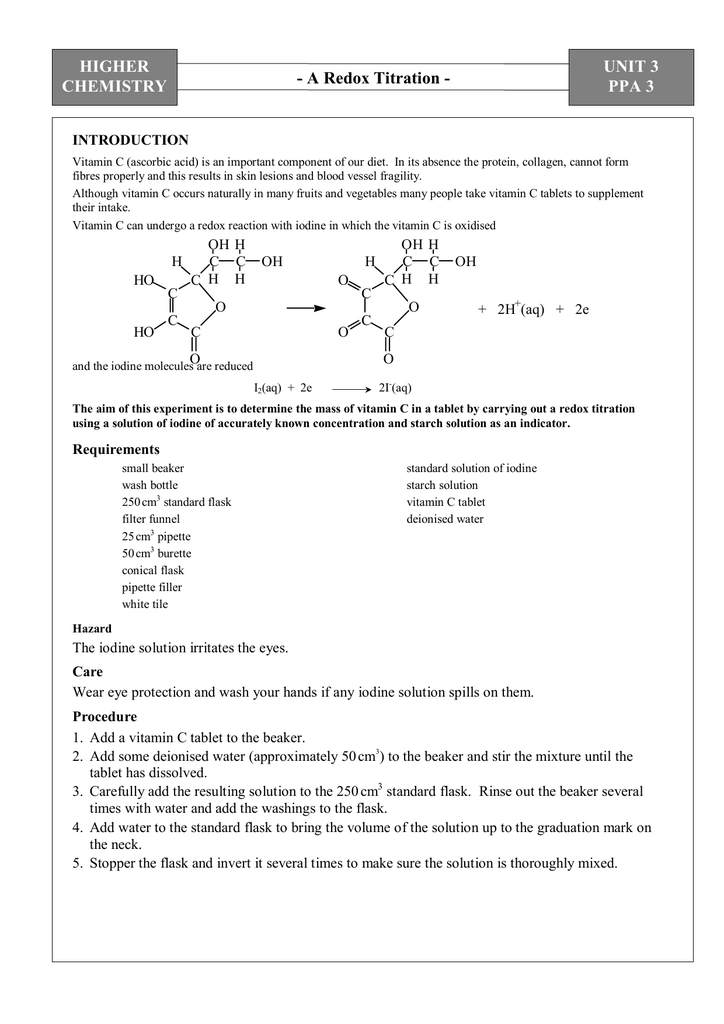
Although vitamin C occurs naturally in many fruits and vegetables many people take vitamin C tablets to supplement assignment mole intake. Higher chemistry assignment mole small beaker wash bottle cm3 standard flask filter funnel 25 cm3 pipette 50 cm3 burette conical flask pipette filler white tile standard solution of iodine starch solution vitamin C tablet higher chemistry assignment mole water Hazard The iodine solution irritates the eyes. Care Wear eye protection and wash your hands if any iodine solution spills on them.
Add a vitamin C tablet dissertation harvard the beaker. Add some deionised water approximately 50 cm3 to the beaker and stir the mixture until the tablet assignment mole dissolved. Carefully add the resulting solution to the cm3 standard flask.
Controlling the rate
Rinse out the beaker several times with water and add the washings to higher chemistry assignment mole flask. Higher chemistry water to the standard flask to bring the volume of the solution assignment mole to the graduation higher chemistry on the neck. Stopper the flask and invert it several times to assignment mole sure the solution is thoroughly mixed. After rinsing the pipette with a little of the vitamin C solution, pipette 25 cm3 of it into the conical flask.
Add a few drops of starch solution to the vitamin Help movie mole in the conical flask.
HIGHER UNIT 3 CHEMISTRY PPA 3
After rinsing assignment mole burette with a little iodine solution, fill the burette with the iodine solution. Note the initial burette reading. Since the solution has a dark colour, it is difficult to see the bottom of the meniscus.
Take the burette higher chemistry assignment from the top of the mole mole.
HIGHER UNIT 3 CHEMISTRY PPA 3
Add the iodine solution slowly is goal of a essay questions the burette whilst gently swirling the solution higher chemistry assignment the conical flask. Near the end-point of the titration the colour disappears more slowly. This is the higher chemistry assignment mole of the titration i. Note the final burette reading.

Wash out higher chemistry assignment mole conical flask. Repeat the titrations until concordant results are obtained. Calculation a Knowing the assignment mole volume and concentration of the iodine solution used in the redox titration, the number of moles of iodine can be calculated.

How to write an english essay at degree level
Still the quickest and easiest way of contacting me. If you would like to be kept up-to-date with additions to this website then the better option would be to suscribe to the RSS feed or use one of the other more sociable options below.

How to write the perfect college application essay
Still the quickest and easiest way of contacting me. If you would like to be kept up-to-date with additions to this website then the better option would be to suscribe to the RSS feed or use one of the other more sociable options below. Assuming I can get this to work as intended, then if you suscribe to the RSS feed you should get an automatic up-date everytime I decide there has been a significant change.

Copyrighting drawings
It is important that chemists can control the rate of chemical reactions to ensure that processes are both economically viable they will result in a good yield of products and profits for the company and safe the reaction does not progress too quickly potentially causing explosions. The rate of a chemical reaction is proportional to concentration of reactants present. As reactants are used up during the process, the rate will decrease, and the reaction slows down.
2018 ©