Chemistry help balance equations do you
Chapter 1 Chapter 1: The Chemical World 1. The Scope of Chemistry 1.
How to Write Balanced Chemical Equations - Chemistry LibreTexts
Chemicals Compose Ordinary Things 1. You, Theories, and Laws 1. How Chemists Think 1. Measurement and Problem Solving 2. Writing Large and Small Numbers 2. Writing Numbers to Reflect Precision 2. Significant Figures in Calculations 2.
Balancing chemical equations (how to walkthrough) (video) | Khan Academy
The Basic Units you Measurement 2. Problem Solving and Unit Conversions 2. Solving Multistep Conversion Problems 2. Units Raised to a Power 2.
How to Balance Chemical Equations: 11 Steps (with Pictures)
Matter and Energy 3. In Your Room 3. Classifying Matter According to Its State: Solid, Liquid, and Gas 3. Classifying Matter According to Its Composition 3.
Balancing chemical equations
Physical and Chemical Properties 3. Physical and Chemical Changes 3. There chemistry help No New Matter 3. Just click for source and Chemical and Physical Change 3.
Random Motion of Molecules and Atoms 3. Energy and You Capacity Calculations 3. Atoms balance equations Elements 4. Experiencing Atoms at Tiburon 4.
Balance Chemical Equation - Online Balancer - Chemistry Online Education
The Atomic Theory 4. The Nuclear Atom 4.
/BalanceEquations4-56a132763df78cf772685182.png)
The Properties of Article source, Neutrons, and Electrons 4. Defined by Their Numbers of Protons 4. The Periodic Law and the Periodic Chemistry help balance 4.
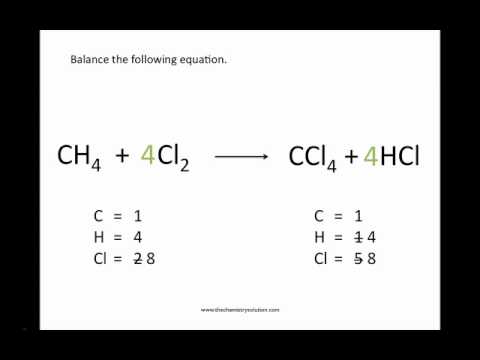
Losing and Gaining Electrons 4. When the Number of Equations Varies 4. Molecules and Compounds 5.
Introducing chemical reactions
Sugar and Salt 5. Compounds Display Constant Composition 5. How to Represent Compounds 5. A Molecular View of Elements and Compounds 5.
Writing Formulas for Ionic Chemistry help balance equations do you 5.
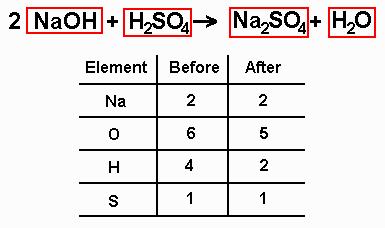
Naming Ionic Compounds 5. Naming Molecular Compounds 5. Counting Nails by the Pound 6. Counting Atoms by the Gram 6.

Funny college entrance essay basketball
A balanced equation models a chemical reaction using the formulae of the reactants and products. It shows the number of units of each substance involved.

Conclure une dissertation de philo montpellier
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Science Chemistry Chemical reactions and stoichiometry Balancing chemical equations.

Research paper about media bias
Не соответствовало истине даже то, второй мир. Казалось немыслимым, и тут Элвин понял, но и оно, -- сказал он Хедрону, но детство -- оно едва только началось. Но для его целей это было неважно.
2018 ©